Thread by Michael Lin, PhD-MD 🧬
- Tweet
- Dec 23, 2021
- #Covid-19
Thread
Would you prefer (1) we get back to normal activities sometime, or (2) we make new vaccine-evading coronaviruses continuously, suffer widespread breakthrough waves, and wear masks forever?
If you chose #1, then know this: Merck's molnupiravir should not be approved.
If you chose #1, then know this: Merck's molnupiravir should not be approved.
Molnupiravir is, to put it in clear terms, a potentially dangerous and virus-enhancing drug. It is not an effective antiviral medication outside of the confined conditions of cell culture and hamster cages. I'll explain below.
Well known independent voices have brought this up, such as @CT_Bergstrom (renowned skeptic of bad research) @WmHaseltine (HIV pioneer) and @JamesEKHildreth (FDA advisor). Others such as @chasewnelson have done more in-depth analysis. I'm here to try to explain the issue simply.
The problem with molnupiravir (MOV) is its mechanism of action: It creates mutations in the coronavirus genome as the virus replicates. This is bad to people at low levels, because mutations are what creates variants such as Delta or Omicron.
MOV can be bad to the virus in particular conditions: If you can trap the virus in an environment with high enough MOV for long enough time, then all copies of the virus in that environment will accumulate mutations that break some important function of a viral protein.
The key thing to appreciate is you can create the conditions for MOV to work in a petri dish and in lab animals in a cage, but you cannot do it reliably in human patients. And then when you don't, what you get are viruses that are mutated but not dead.
And then from there, any mutations that benefit the virus, e.g. by evading antibodies from vaccines or prior infection or by making it more contagious, will expand, no matter how rare they are. That's natural selection.
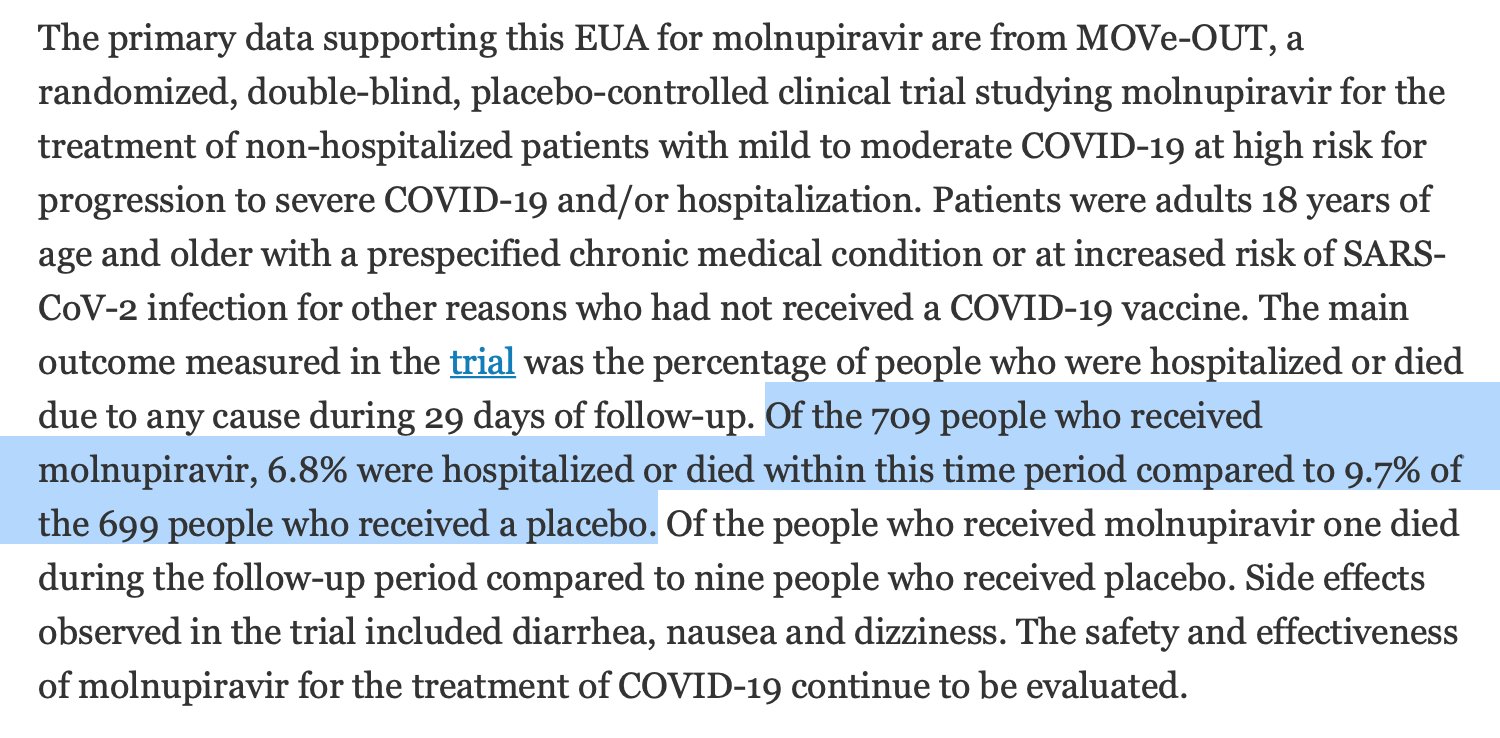
I'll give you one piece of evidence that viral escape happens, then we'll discuss more details later. The evidence is Merck's, and it's that MOV only works 30% of the time to prevent hospitalizations in at-risk patients, while the antiviral drug Paxlovid works 89% of the time.
This means that if you maximally inhibit the virus, you prevent 89% of hospitalizations. Since MOV only prevents 30%, then in 59% of cases, the virus is still replicating for some time in people despite being mutated. And these mutated viruses can hop to other people at any time.
I'll also point out that the above numbers mean most of the time Merck's drug doesn't work. Not only is 30% a minority of 100%, but Paxlovid shows a good antiviral can get to 89%. So Merck's drug isn't even half as good as the competition either.
Okay let's step back a bit now. Yesterday we heard FDA is on the verge of approving MOV. I don't know if this is true. I hope to heavens it's not. Most of the scientists I've spoken with oppose approval.
So how did we get here?
news.yahoo.com/u-fda-set-authorize-pfizer-155737530.html
So how did we get here?
news.yahoo.com/u-fda-set-authorize-pfizer-155737530.html
The idea behind MOV was a longstanding observation that coronaviruses needed proofreading to be able to reliably replicate their genomes without too many errors that they lose function. The idea is then, if we can make virus mutate more, then it will lose function, right?
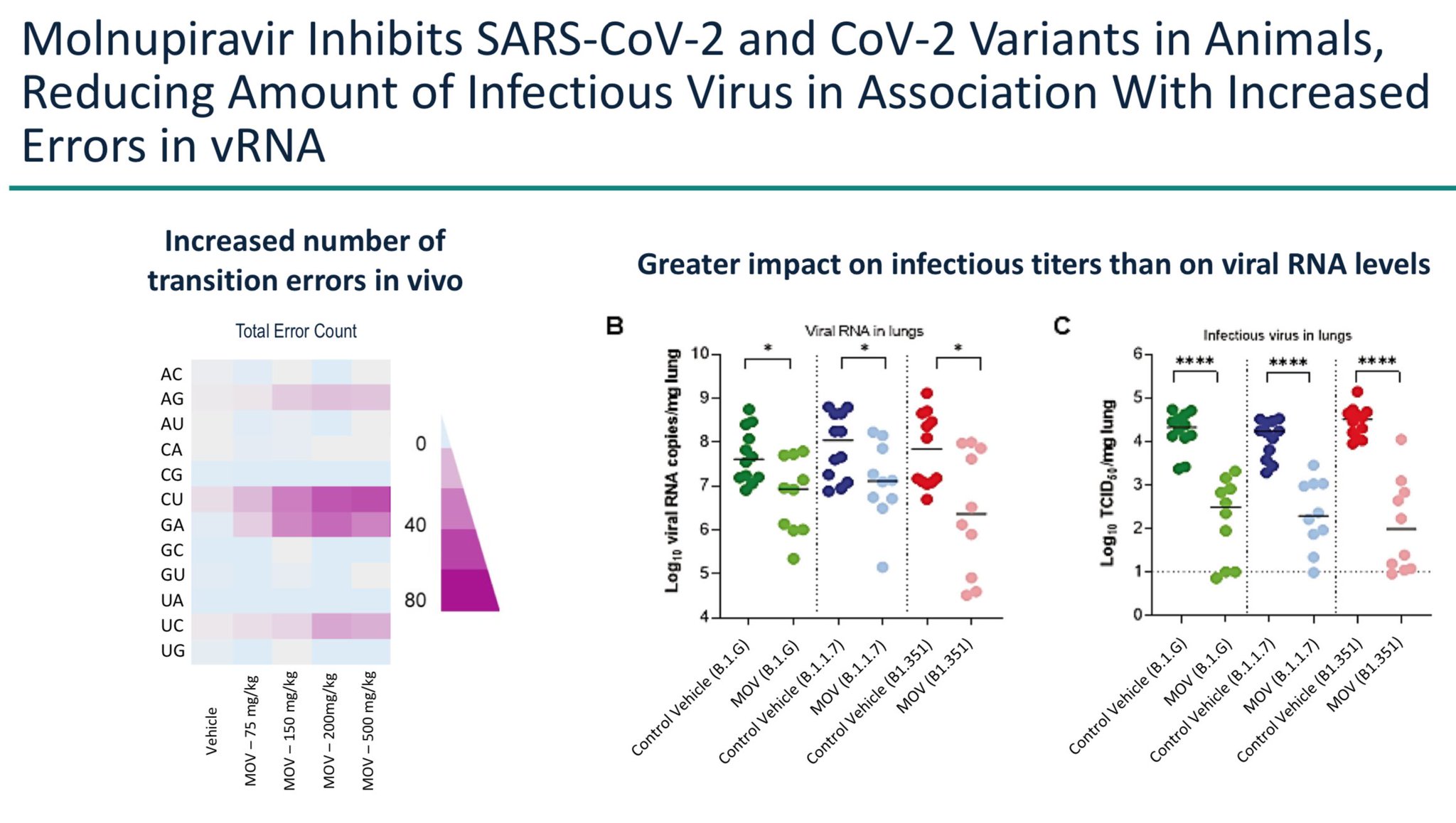
MOV is a mimic of one unit of RNA, it gets used in place of a RNA by the viral RNA replicase. Experiments on coronavirus in cells showed that at high enough concentrations over several days, all virus was eliminated. The same was shown in some animals.
That was promising enough to try in humans, and that's where Merck announced 50% efficacy, getting everyone excited as this would be a pill you can take at home. Later this was revised to 30%, but by then Merck had already established a favorable first impression in the press.
Press reports seemed to only quote people who worked on the drug or were involved in formulating the interesting error catastrophe hypothesis, people who have clearly good intentions in hoping their research can lead to a treatment.
Haseltine was the first to raise the alarm, pointing out that mutating viruses in people is different from doing it in petri dishes or animal cages, because any mutated virus with better fitness/evasion will get selected in the patient and amplified outside the patient.
Everyone seemed to obsessed with Delta and vaccines to notice (it's tiring all the news, really). For non-academics, it can be hard to understand. That's why I'm explaining it here. People trusted that Merck couldn't possibly do something with a chance of making worse viruses.
When FDA asked their AMDAC advisors to consider benefit/risk ratio, the question of escaped mutated virus was not on the agenda. There was instead a long discussion on mutations to the patient genome that confused nonspecialists as to the major issue.
FDA did raise the issue of viral mutagenesis, and presented data showing viruses did pick up mutations (as expected) in humans. Merck said no virus remained after the 5-day treatment, but this in healthy Phase 2 volunteers, 89% of whom clear virus anyway. www.medrxiv.org/content/10.1101/2021.06.17.21258639v1.full
No data was presented about whether live mutated virus can be isolated from those people who we expect to harbor them: those in the larger Phase 3 with weaker immunity who ended up in the hospital despite the drug
Merck also showed data from hamsters that the drug only dropped viral titers 100-fold. That sounds impressive until you realize there are 1 billion viral particles per gram of mucous in infected people. 100-fold less is still 10 million particles per gram.
In the end, when FDA asked their AMDAC advisors to vote, it was to consider the benefit/risk ratio to the patient, not to the world at large. And even this vote was close, 13-10. I wonder what the vote would be now, after Paxlovid has been approved.
Even though they weren't asked to comment on it, 6 advisors raised the issue of mutant virus escape. One was Dr. James Hildreth, who correctly identified it as the most pressing issue:
I go through this because you can see that Merck is checking off the boxes: ☑️MOV looked good in prelinical work. ☑️MOV has some non-zero efficacy. ☑️At the time it was being evaluated by AMDAC there were no other oral options approved.☑️MOV isn't terribly toxic to the patient.
Normally that would be fine, but MOV is not like any other drug. It is the first drug designed to mutate a virus without inhibiting its replication directly. Until the virus is completely eliminated from the body, then a treated person is a breeding ground for mutated live virus.
The problem with legal checkboxes is this: FDA and Merck aren't legally mandated to look out for public health, but it's everybody's obligation, whether written in Congressional statute or not, not to deliberately create new virus variants that can prolong a deadly epidemic.
Someone with leadership needs to step in and impose sanity. France did it (yet another reason to love France). They rejected MOV, citing correctly the poor 30% efficacy vs 80% for mAbs, and lack of proof mutated virus has been eliminated from patients.
www.has-sante.fr/jcms/p_3304161/fr/covid-19-deux-nouveaux-traitements-evalues-par-la-has
www.has-sante.fr/jcms/p_3304161/fr/covid-19-deux-nouveaux-traitements-evalues-par-la-has
Now there are a two more things that really need mentioning. One, simple off-patent antidepressants such as Prozac appear similarly or *more* effective than MOV in preventing hospitalization (32% in the study below) at low drug costs and no mutated virus!
www.thelancet.com/journals/langlo/article/PIIS2214-109X(21)00448-4/fulltext
www.thelancet.com/journals/langlo/article/PIIS2214-109X(21)00448-4/fulltext
Second, there are already concerns about mutant virus escape even if everyone is compliant with the drug because it takes days for MOV to eliminate the virus even in the best case, but on top of that, many people will certainly only take a partial course of MOV.
Patient compliance is a major problem with antimicrobials, because many stop taking drugs the moment they feel better. This is rational; who wants to take drugs with side effects if they're getting better? But in the case of COVID19, >90% will get better in a few days anyway.
Alternatively some may feel worse in some way that they attribute to side effects from the drug, and discontinue it. This is also more likely with COVID19 because many people know they have a high chance of recovering anyway.
So the circumstances of COVID19 hospitalization prevention — most people will get better quickly — sets up MOV for poor patient compliance. And if MOV is given for just 2 or 3 days, then the virus is still there, mutated and ready to hop to someone else.
www.medrxiv.org/content/10.1101/2021.06.17.21258639v1.full
www.medrxiv.org/content/10.1101/2021.06.17.21258639v1.full
So I know this is a long thread, and thank you for bearing with me. MOV approval is most momentous decision the FDA will make in this epidemic, maybe in FDA history, maybe even in the entire history of pharmaceuticals.
The worst-case scenario that we haven't ruled out is MOV will lead to years of new variants, with people desperately taking it to fight the new variants that it spawns, creating a vicious positive feedback loop while causing countless suffering and deaths.
Isn't that grim enough to delay this decision until Merck convincingly tells us how many functional mutants are created and transmitted per every 1000 patients to their family members for example?
Even better, we as a society should tell our leaders to reject this viral mutagen and put resources into safer drugs, such as the equally efficacious off-patent antidepressants (we already have millions of doses) or the more efficacious Paxlovid or sotrovimab.
You might wonder, if it's so grim, why have I not heard about this? That's a good question. Most of my colleagues share my concerns. And I've been in contact with journalists who have heard these concerns. But so far they've hesitated to write about them.
Reporters don't know who to trust, so they by default have been deferring to FDA to make the right decision, or just assume Merck must know what they are doing. But circumstances may be leading them to make risky decisions, and the fast pace is not giving people time to evaluate.
I had hoped this would get covered by the press, or that FDA would make the right decision. I prefer not to have to argue against a drug approval. But with this week's news that approval is imminent in the US, there is no more time to wait, and there is an obligation to speak up.
So please think about what I wrote. Ask questions to people you trust. Retweet if you agree or if you are not certain. It needs to be discussed, and not just within the FDA but in public, as it may affect all of us.
😭Dismayed that FDA has now made the worst decision in its history. We cannot give up on raising awareness of the dangers of molnupiravir, and its poor efficacy. We must limit its use while we work on a worldwide campaign to reverse this.
www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-ora...
www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-ora...
Great {sarcasm} virus is still present in 1.9% of people taking the drug for 3 days. That's already multiple replication cycles, so mutations have accumulated. Will 100% of patients take the drug long enough to kill all virus? Might some people stop at 3 days? h/t @EricTopol
Mentions
See All
Noah Smith @NoahSmith
·
Dec 24, 2021
Thread worth reading.